TAILWIND®
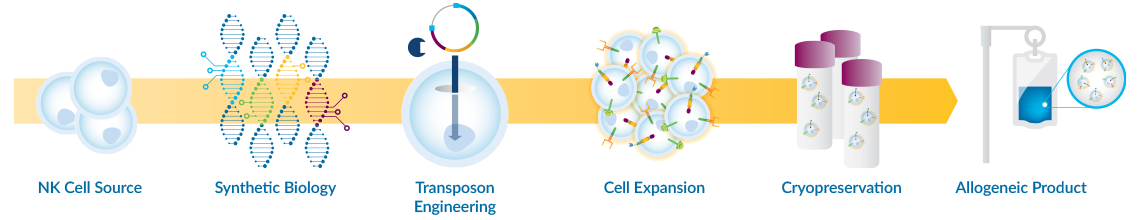
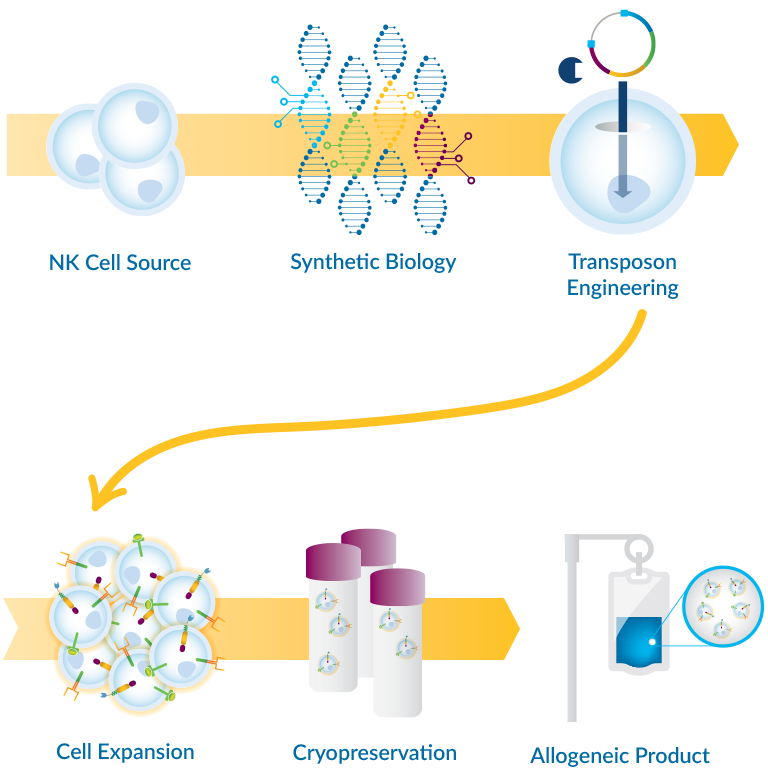
TAILWIND® is a proprietary integrated suite of technologies to engineer and manufacture NK cells into safe, effective and off-the-shelf cell therapy products to treat solid tumors. NK cells are innately cancer fighting and are attractive for allogeneic cell therapy products to address the unmet needs of many cancer patients. To overcome the immunosuppressive tumor microenvironment (TME), we use the power of synthetic biology and innovative non-viral cell engineering to program new functionalities into NK cells, ultimately creating chimeric antigen receptor-NK (CAR‑NK) cells with enhanced cancer-killing properties.
Key attributes of TAILWIND® for differentiated CAR-NK cell therapies:
- Transposon engineering of NK cells for more efficient manufacturing
- Synthetic biology-enabled modular switches to address TME immunosuppression
- CAR architecture optimized for NK cells
Transposon engineering of NK cells for more efficient manufacturing
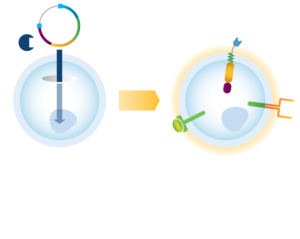 We are pioneering the application of non-viral NK cell engineering with the TcBuster Transposon System to efficiently produce potent CAR-NK cell therapies. Unlike conventional viral methods, transposon systems deliver large genetic payloads and enable single-step, multiplex cell engineering. These features allow versatile programming of CAR-NK cells and a more cost-effective, streamlined manufacturing process. Using the TcBuster Transposon System, we equip our CAR-NK cell therapies with functional attributes not found in earlier generations of cell therapies.
We are pioneering the application of non-viral NK cell engineering with the TcBuster Transposon System to efficiently produce potent CAR-NK cell therapies. Unlike conventional viral methods, transposon systems deliver large genetic payloads and enable single-step, multiplex cell engineering. These features allow versatile programming of CAR-NK cells and a more cost-effective, streamlined manufacturing process. Using the TcBuster Transposon System, we equip our CAR-NK cell therapies with functional attributes not found in earlier generations of cell therapies.
Synthetic biology-enabled tumor microenvironment (TME) switches
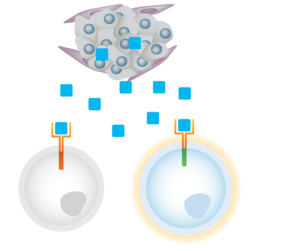 We are building a broad portfolio of TME switches–from signal traps to inverters–tailored to overcome specific immunosuppressive signals presented by solid tumors. Our signal inverters include sense-and-respond switches that are designed to convert immunosuppressive signals into NK-cell activation signals. Our first generation TME switch addresses TGFβ, an immunosuppressive cytokine found in a majority of solid tumors.
We are building a broad portfolio of TME switches–from signal traps to inverters–tailored to overcome specific immunosuppressive signals presented by solid tumors. Our signal inverters include sense-and-respond switches that are designed to convert immunosuppressive signals into NK-cell activation signals. Our first generation TME switch addresses TGFβ, an immunosuppressive cytokine found in a majority of solid tumors.
CAR architecture optimized for NK cells
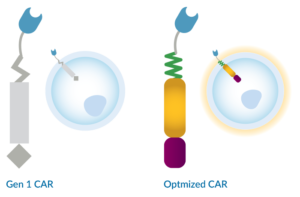 We are developing more effective CAR-NK cell therapies by refining the CAR architecture for optimized function in NK cells. We are employing innovative methods to generate and evaluate novel combinations of CAR elements to achieve robust and enduring cell activation.
We are developing more effective CAR-NK cell therapies by refining the CAR architecture for optimized function in NK cells. We are employing innovative methods to generate and evaluate novel combinations of CAR elements to achieve robust and enduring cell activation.
In addition to generating more potent CAR-NK cells, TAILWIND® integrates the required technologies and capabilities across the full continuum of CAR-NK cell therapy process development and manufacturing, including NK cell isolation, engineering, cell expansion, and cryopreservation.